Rechargeable lithium sulfur batteries have attracted great interest in recent years because of their high theoretical specific energy, which is several times that of current lithium-ion batteries. Compared to sulfur, fully-lithiated Li
2S represents a more attractive cathode material because it enables pairing with safer, lithium metal-free anodes.
Stanford researchers have designed and tested a new framework for a rational design of stable and high performance Li
2S cathodes by using
ab initio simulations to elucidate the interaction between Li
2S and lithium polysulfides with various functional groups found in macromolecular binders. Using polyvinylpyrrolidone (PVP) as a binder in one embodiment, an initial specific capacity of about 760 mAh g
-1 of Li
2S (~1,090 mAh g
-1 of S) was achieved at 0.2C, with unprecedented capacity retention of about 94% in the first 100 cycles. Even after prolonged cycling over 500 charge/discharge cycles, cells retained about 69% of their initial capacity, which corresponds to a small capacity decay of about 0.062% per cycle.
Figure 1 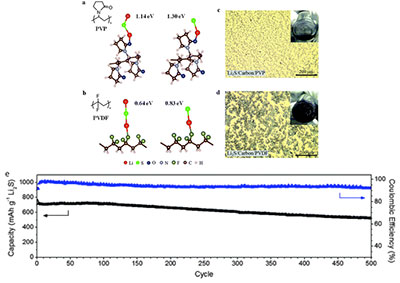 Figure 1 description
Figure 1 description -(a, b) Ab initio simulations showing the most stable configuration and calculated binding energies of Li
2S and Li–S• species with (a) PVP and (b) PVDF binders. (c and d) Optical microscopy and digital camera images (inset) showing the electrode slurry of (c) Li
2S /carbon black/PVP binder and (d) Li
2S /carbon black/PVDF binder in N-methyl 2-pyrrolidinone (60:35:5 by weight in both cases) (e) Specific capacity and Coulombic efficiency of Li
2S cathodes using PVP binder upon prolonged cycling over 500 cycles at 0.2 C.
Stage of Research Demonstrated best cycling performance for Li2S cathodes to date.
Results showed stable and high-performance Li2S cathodes by using ab initio simulations to guide our rational selection of poly(vinylpyrrolidone) binder which exhibits strong affinity with both Li2S and lithium polysulfides.
A high discharge capacity of 760 mA h g-1 of Li2S (~1090 mA h g-1 of S) was achieved at 0.2C with stable cycling over prolonged 500 charge/discharge cycles.