Researchers at Stanford University and SLAC National Accelerator Laboratory have developed a new coating design which makes lithium metal batteries stable and promising for further development. This new coating forms an artificial solid electrolyte interface (SEI) to prevent cracking and formation of dendrites, preventing fires and explosions.
The team integrated dynamic flowability, fast single-ion conduction, and electrolyte-blocking property into a single matrix, the dynamic single-ion-conductive network (DSN), to obtain a multifunctional coating material. The DSN helps to form a stable protection layer on Li surface, to lower the interfacial impedance and make the cycle life longer for Li metal anodes. This is believed to be the first example of a multifunctional coating layer in a single chemical structure/polymer. With the DSN, the team achieved long cycle life for lithium-metal full cells in commercial carbonate electrolyte. In addition. the solution processability of DSN enables large-scale fabrication.
This invention has the potential to provide a new path for developing practical lithium-metal batteries.
Related Technologies:Stanford docket S15-458 - "Thermoresponsive Material to Prevent Battery Fire"
This invention is a high-performance, ultrafast, thermoresponsive polymer that can act as a circuit breaker to prevent fires in next-generation high-energy-density batteries by rapidly and reversibly turning off when overheated.
Figure: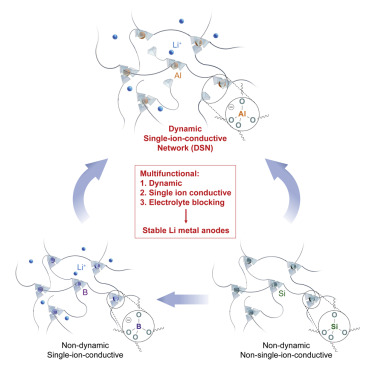
Figure Description - Graphical Abstract-multifunctional material to improve the stability of lithium-metal anodesStage of Development:
- Refining coating design to increase capacity retention and testing cells over more cycles
- After 160 cycles, these Li metal cells still delivered 85 percent of the power as in their first cycle, as compared to 35% in current Li metal cells.